ENGLEWOOD, Colo. (KDVR) — You’ve probably never heard of AYTU Bioscience but stock for the Englewood company jumped 700% earlier this week after it announced the ability to provide rapid test results for the COVID-19.

“I think it’s a game changer in many ways,” said Josh Disbrow, the CEO of AYTU Bioscience. His company has partnered with a Hong Kong manufacturer to produce test kits that can provide results in 2-10 minutes.
“Very few tests have been FDA-approved despite the big need. Tests are getting approved but rapid point-of-care tests have not yet been approved. So if this can get approved, it would be among the first, if not the first,” he said.
Disbrow said AYTU Bioscience has already ordered more than a 100,000 kits for distribution across the United States.
He says if the company gets fast-track approval from the Food and Drug Administration, “The FDA does have a provision whereby products like this and in times like this can go through emergency use authorization and we’ll pursue that angle.”
Disbrow said even with FDA approval, it would take at least a week or two to bring the test kits to the United States. The kits are identical to to those China has been using for weeks.
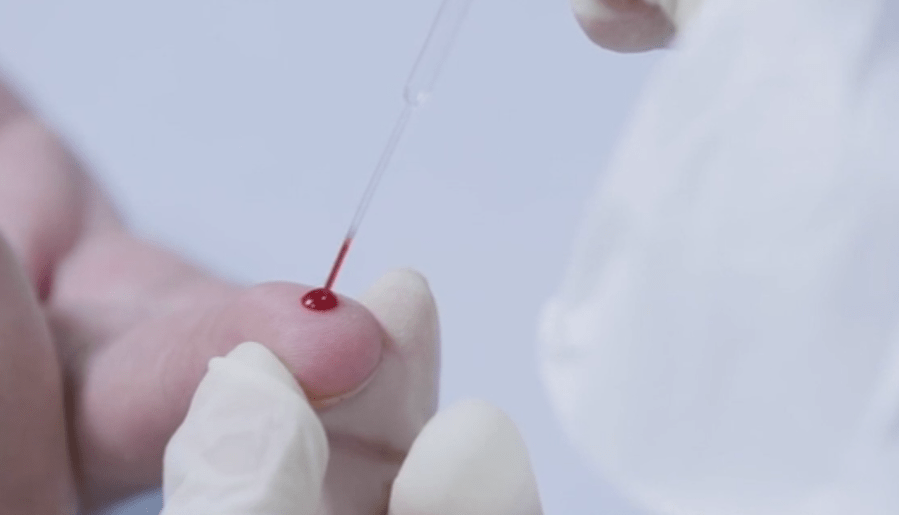
Currently, most Americans are tested using a nasal swap that produces results in 48 hours. The rapid tests use a drop of blood from a finger prick to identify COVID-19 antibodies.
“So the idea [is] that you could get something as quickly as 2-10 minutes to very quickly get sequestered, get quarantined, potentially get treatment. But really, detection is the critical piece right now and time is of the essence,” said Disbrow.
The test is 90% accurate as a screening method and can be performed in a doctor’s office.
